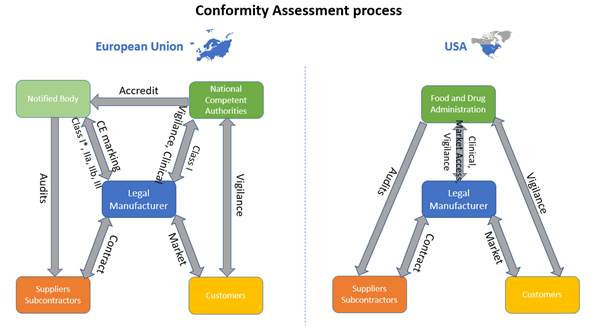
What are the principal differences between the conformity assessment process of a medical device in the USA and in the European Union? - Kvalito

GMED, Notified Body under Regulation (EU) 2017/746 | LNE, Laboratoire national de métrologie et d'essais







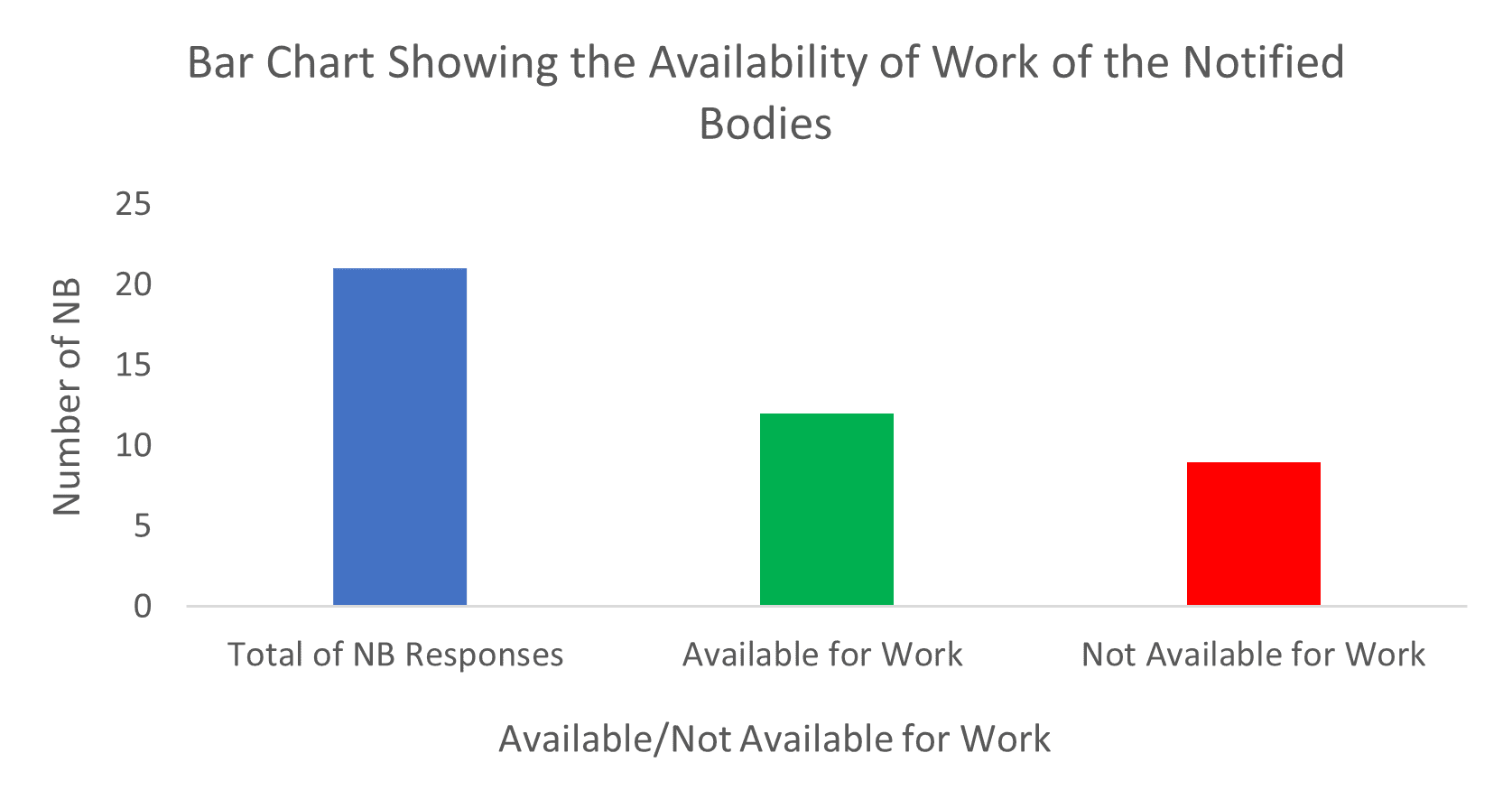
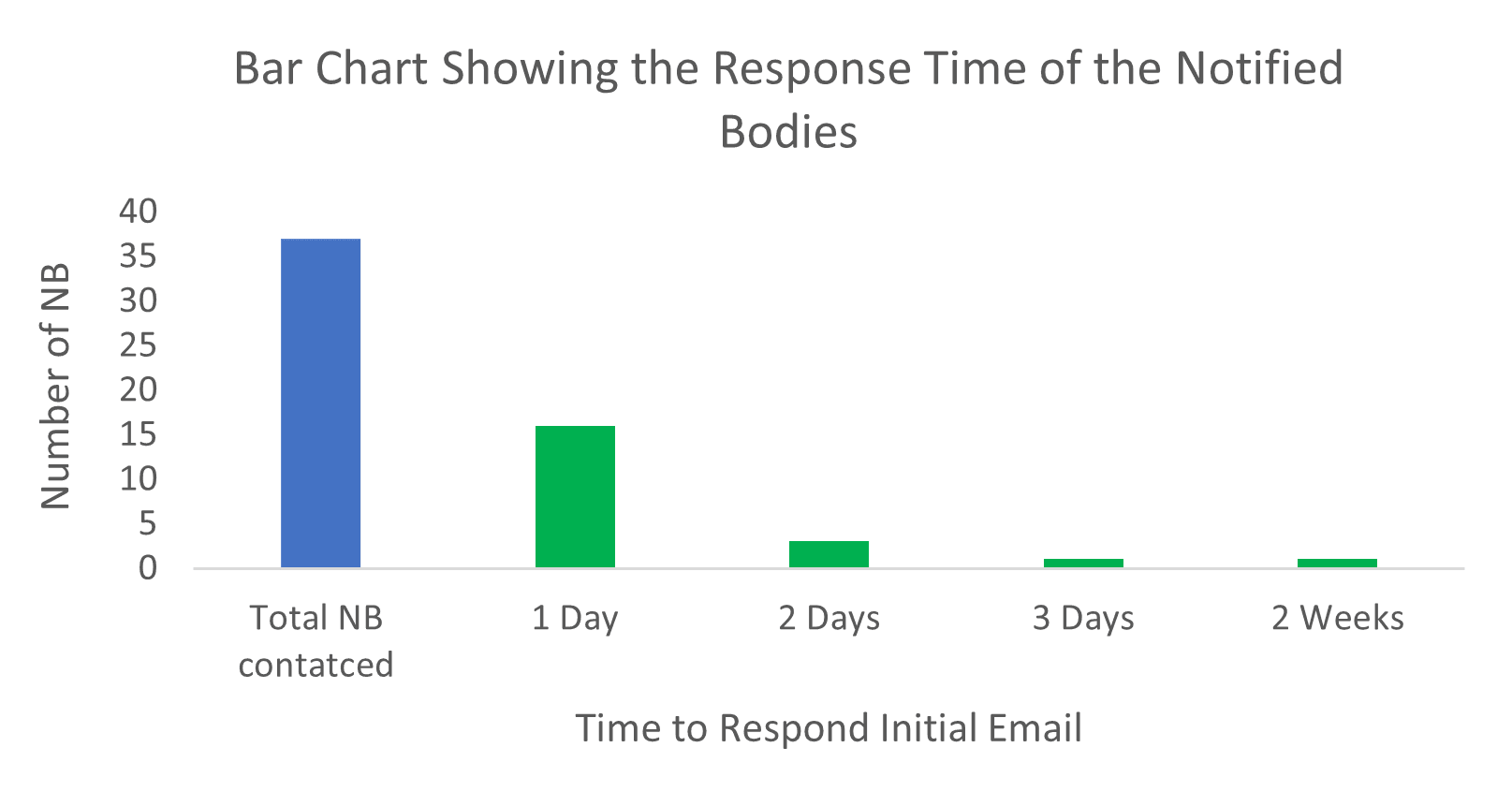

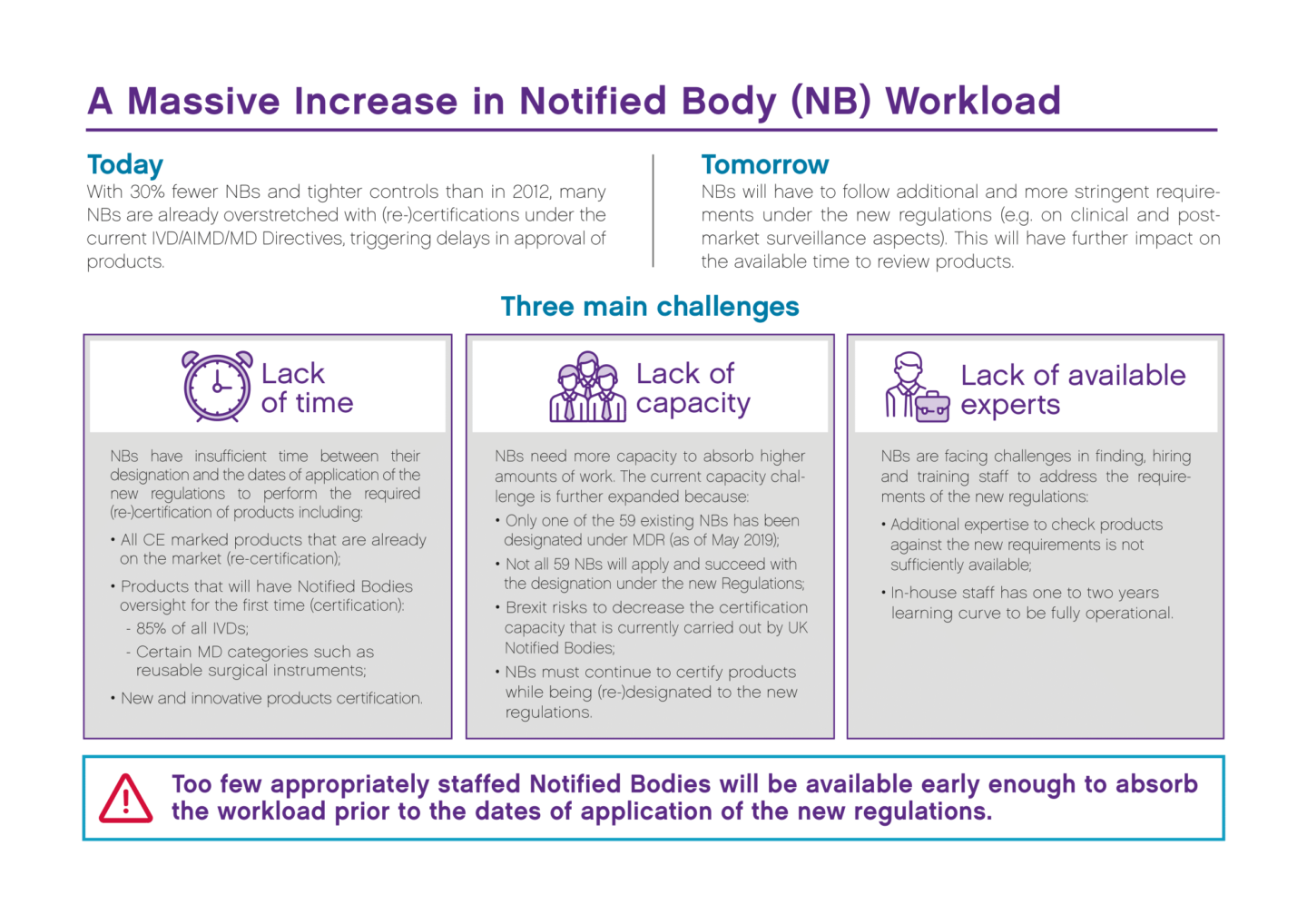



/tuv-rheinland-ivdr-visual-1-en.png)